Malaria is a life-threatening disease with a big global impact. In 2017 there were around 220 million cases of malaria affecting people living in 90 different countries around the world, and causing an estimated 435,000 deaths. Most deaths occur in children under the age of five in sub-Saharan Africa and are caused by Plasmodium falciparum – the most deadly type of malaria parasite.
Malaria can be tackled by either prevention or with treatment. Because malaria is passed to people via infected mosquitoes, people can protect themselves from the disease by preventing mosquito bites – either by spraying insecticides or sleeping under a treated bed net. If that doesn’t work and people become infected, doctors can treat malaria with relatively cheap drugs (where they are available).
However, the speed at which the parasites become drug-resistant has reduced the effectiveness of both insecticides and antimalarial drugs, so efforts have focused on other strategies including vaccination. An effective vaccine against malaria could have a huge impact on the disease, yet progress has been slow – despite intensive efforts from researchers and industry over many years.
Currently there is only one vaccine for malaria that has reached the last stages of development, RTS,S (Mosquirix), which gives some infants (up to 17 months old) partial protection against malaria. But infants need 4 injections to have the best chance of being protected, and immunity isn’t long-lasting.
There is an urgent need for more effective vaccines as part of a coordinated plan to reduce and eventually eliminate malaria. But as we’re about to discuss, making an effective vaccine isn’t a simple task.
Aiming at a moving target
One of the main reasons vaccine design is so challenging is that like many parasites, malaria has a complicated, multi-stage life cycle.
Malaria relies on two hosts – mosquitos and human – for its survival and reproduction. During these two phases the parasites completely change their form.
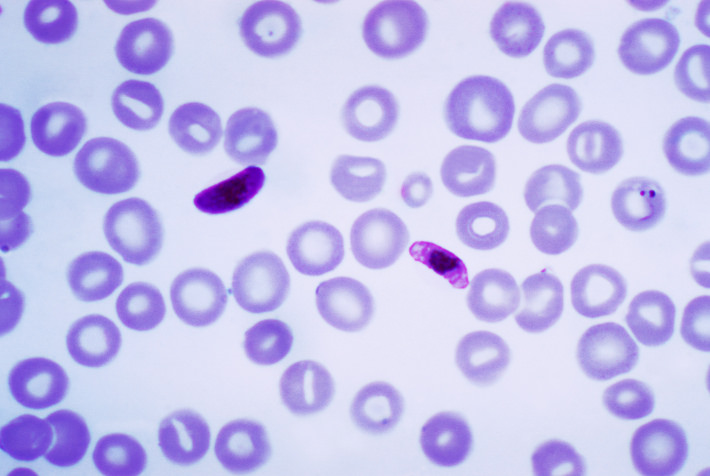
Image courtesy of Centers for Disease Control and Prevention, (Wikimedia Commons)
The life-cycle in people begins when they are bitten by an infected mosquito and immature parasites, called sporozoites, enter the body. The sporozoites travel to the liver and infect cells there, maturing into adult parasites called merozoites over seven days. The liver cells burst open and release them into the blood, where they undergo repeating cycles of entering red blood cells, multiplying (causing the red blood cells to burst), and entering new red blood cells.
These waves of parasites released into the blood stream cause the symptoms associated with malaria – fever, chills, sweating, headaches, nausea, tiredness and aching. In more serious cases, the parasites can affect the brain (cerebral malaria), cause anaemia, stop the lungs taking in oxygen properly, prevent blood clotting or cause blood vessels to collapse, or lead to organ failure.
Some of the parasites leave this red blood cell cycle and change form again into male and female gametocytes (like human egg and sperm) – this is the first stage of sexual reproduction. When the next mosquito bites an infected person to take a blood meal, some of the gametocytes can end up in the gut of the mosquito. Male and female fuse making new “baby” parasites – these sporozoites travel to the mosquito’s salivary glands, ready to infect another person and start the process all over again.
As the malaria parasites switch between their immature, mature and sexually reproductive forms and move between people and mosquito hosts, they change size, shape, and the diverse array of proteins covering their surface.
Their variety and changing characteristics make it very challenging to develop a vaccine. One tactic researchers are trying is combining targets to different stages of the parasite’s life-cycle in one vaccine, so the immune system can spot and kill the parasites at any point in their life cycle.
Trying a combination of vaccines
Research published in October 2018 in NPJ Vaccines, led by Professor Adrian Hill based at Oxford University, tested the RTS,S vaccine in combination with a newer vaccine, ME-TRAP, that works in a different way.
One of the researchers, Dr Katie Ewer, explains the difference between the vaccines. “The RTS,S vaccine targets the parasites when they first enter a person’s blood – the sporozoite form. It works by triggering the immune system to make antibodies that latch onto the parasites and stop them from getting into the liver.”
“The other vaccine, ME-TRAP, makes use of harmless viruses to stimulate the immune system to produce an army of killer white blood cells that can recognise and destroy malaria parasites growing in the liver. This could stop the malaria merozoites being released into the blood and making people ill,” says Dr Ewer.

The viruses used in the ME-TRAP vaccines have been widely tested as a tactic for lots of other vaccines – they can’t grow on their own inside people and have been shown to be very safe in adults and children.
The team already had results showing that giving people the two different vaccines close together made them more effective than either vaccine individually, giving people better protection against malaria. But this meant giving 5 separate injections, which isn’t practical for a wider vaccination programme.
“We wanted to combine the vaccines and give them at the same time, and find out if this worked as well,” says Dr Ewer.
A malaria human infection study
The researchers used a human infection study, whereby volunteers are purposefully infected with malaria in a controlled and safe way, to get some early results about the new vaccine combination.
“Around 40 volunteers who had agreed to take part in the study were spilt into different groups. Some weren’t vaccinated, the other groups were given 3 doses of the RTS,S vaccine alone or combined with the new vaccine,” says Dr Ewer. “Then 3 weeks after the last vaccine, they each received 5 bites from mosquitos infected with a known strain of malaria.”
The malaria strain used in the study is sensitive to a drug called chloroquine, meaning the infection could be easily treated. 6 days after being bitten by the mosquitoes, the volunteers attended a clinic so the team could monitor them for signs of malaria and take blood samples to test.
“If anyone had any symptoms, for example a raised temperature, or we saw parasites in their blood sample, the volunteers were given immediate treatment,” says Dr Ewer. “The study ended after 21 days, and even if there were no signs of malaria, all our volunteers had the anti-malarial drug at this point. We then checked the drugs had worked by examining blood samples so we knew all the volunteers were free from the parasite at the end of our study.”
By experimentally exposing volunteers to malaria, the researchers were able to study how effective the new vaccine combination was at protecting people from malaria.
A surprising result
Although giving the two vaccines separately increased their effectiveness, when they were given at the same time the two vaccines didn’t work as well as the RTS,S by itself.
“Although combining vaccines that target the immune system against different stages of the malarial life-cycle is a good idea, hitting the infection on multiple fronts, in this case it didn’t work,” says Dr Ewer.
When the researchers did some thorough analysis of the blood samples taken from the volunteers during the study, they found that the harmless viruses in the new ME-TRAP vaccine changed the way the immune system reacted to the RTS,S vaccine.
“The presence of viruses in the ME-TRAP vaccine changed the chemical signals immune cells send to each other and the kind of attack they can mount against the parasites. It meant that the RTS,S vaccine didn’t generate antibodies in the same way, reducing how well it worked.”
Although disappointing, the results reveal important information about how the different vaccines interact with the immune system and why this particular combination didn’t work.
“Combining vaccines that target different stages of the life-cycle of the malaria sporozoite is still a good approach to getting a better vaccine, but this study showed that it needs careful testing to get the best result,” says Dr Ewer.
With millions of children’s lives at risk from malaria, research into an effective vaccine programme is crucial. The EU has funded a new 5-year research program at the University of Oxford to continue their important studies of different combinations of malaria vaccines. Human infection studies, and the goodwill of the volunteers giving up their time to take part, will continue to play a central role in testing these new combinations.
Emma
Rampling T, Ewer KJ, Bowyer G, Edwards NJ, Wright D, Sridhar S, Payne R, Powlson J, Bliss C, Venkatraman N, Poulton ID, de Graaf H, Gbesemete D, Grobbelaar A, Davies H, Roberts R, Angus B, Ivinson K, Weltzin R, Rajkumar BY, Wille-Reece U, Lee C, Ockenhouse C, Sinden RE, Gerry SC, Lawrie AM, Vekemans J, Morelle D, Lievens M, Ballou RW, Lewis DJM, Cooke GS, Faust SN, Gilbert S, Hill AVS, Safety and efficacy of novel malaria vaccine regimens of RTS,S/AS01B alone, or with concomitant ChAd63-MVA-vectored vaccines expressing ME-TRAP, NPJ Vaccines. 2018 Oct 9;3:49. doi: 10.1038/s41541-018-0084-2